Dierckx (1901)
What is Penicillium corylophilum, and where can it be found?
Penicillium corylophilum is a fungus belonging to the subgenus Furcatum of the Penicillium genus [1]. It can commonly be isolated from cereals (barley, paddy rice, wheat), flour, nuts, frozen fruits and cakes, dairy, fruit, and meat products. P. corylophilum often contaminates high-fat foods, such as rapeseed, rapeseed oil, and margarine [1, 2]. It is also frequently found in damp buildings, moldy building materials, wood, and manufactured wood [2]. Studies have shown that P. corylophilum comprised about 5 % of the Penicillium molds isolated from damp buildings in Scandinavia, West Europe, and Japan [2]. This species is also considered entomopathogenic, meaning it parasitizes insects such as houseflies and mosquitoes [3, 4, 5].
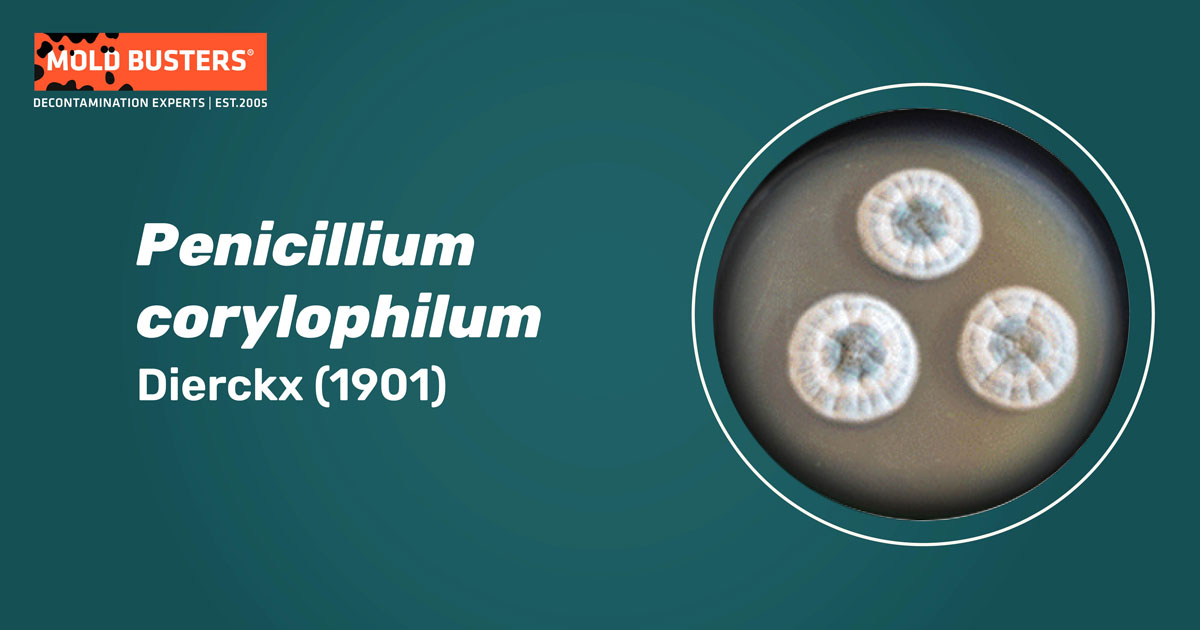
How does Penicillium corylophilum look like?
Penicillium corylophilum is xerophilic, meaning it can live in environments with low availability of water (substrate water activity) [1].
In the laboratory, it is commonly grown on Czapek Yeast Autolisate Agar (CYA) or Malt Extract Agar (MEA), which produces colonies that are low, velutinous, white to dull green with a dark center (Fig. 1) [1]. Penicillium corylophilum cannot grow at 37°C (98.6 °F) nor germinate at 5°C (41 °F) [1].
Substrate mycelia are white, producing smooth conidiophores that bear two or three unequal branches [1,6]. Conidia are spherical to ellipsoid, 2.5–3.0 mm (0.09–0.12-inch) in diameter, smooth-walled, and arranged in irregular chains or long columns [1,6]. The distinctive characteristics of P. corylophilum are irregular, long, divergent metulae (branches of conidiophores bearing conidia) (Fig. 1) [1].
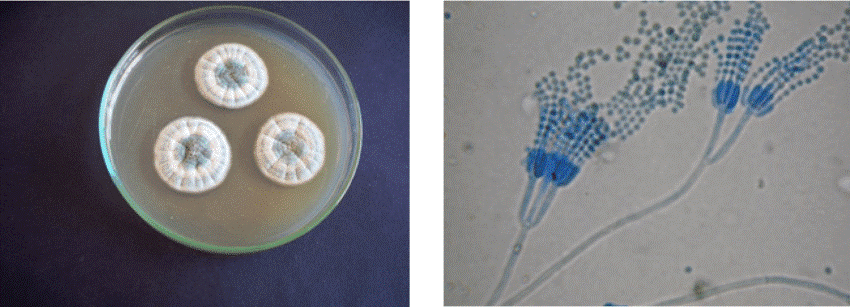
(Image source: Elgarhi, H. et al. 2020)
What are health effects of Penicillium corylophilum?
A large number of Penicillium spores are present in the air at any moment. However, Penicillium sp. infections are rare compared to commonly reported infections caused by the closely related aspergilli [7]. Infections caused by some Penicillium species are usually associated with injuries, surgery, or other invasive procedures, as well as with contaminated prosthetic materials [7]. On the other hand, allergic reactions to Penicillium species (bronchopulmonary penicilliosis) are relatively common, mostly among asthmatic and atopic individuals [7]. Hypersensitivity to penicillin with pulmonary manifestations is also related to certain occupations. It is commonly referred to as “cheese worker’s lung”, “peat moss worker’s lung”, “malt worker’s lung”, or “farmer’s lung” [7]. Penicillium species are also related to the “sick building syndrome” due to the high spore count and hyphal fragments in the indoor air [2]. According to studies, hypersensitivity is caused by Penicillium metabolites such as triple-helical β-(1, 3)-D glucan and low molecular weight compounds [2]. Superficial infections caused by penicillin include nail infection (onychomycosis), skin inflammation (dermatitis), eye and ear infection [2].
P. corylophilum is one of the most commonly isolated species in damp buildings and crawl spaces [2, 8]. Although this species has been reported to produce allergens related to a serious lung disease called hypersensitivity pneumonitis [2], no significant environmental health risk is connected to it [8]. However, P. corylophilum is one of thirty-six fungal species included in ERMI testing (The Environmental Relative Moldiness Index), developed by the U.S. Environmental Protection Agency [9].
Toxicity of Penicillium corylophilum
Penicillium species can have adverse effects on human health due to their production of certain secondary metabolites [7]. Secondary metabolites are compounds produced when the active growth of fungi has ceased, so they are not directly related to fungal growth, development, and reproduction. They include antibiotics, alkaloids, mycotoxins, and plant growth factors such as gibberellins [10].
Penicillium corylophilum is known to synthesize numerous secondary metabolites. Some exhibit antibacterial activity [11], while others are known for their toxic effects on mosquitoes and other insects [4, 5]. Penicillium corylophilum also produces very harmful compounds such as mycotoxin citrinin and ergot alkaloid epoxyagroclavine [2, 10].
Citrinin (CTN) is initially isolated from Penicillium citrinum, which is the major producer of this mycotoxin from a group of polyketides. It has been reported that other Penicillium species, including P. corylophilum, can produce citrinin [10]. Citrinin is found in different plant products, mainly in grains after harvest and stored grains [12]. It also occurs in beans, vegetables, fruits, herbs, and spices, often with other mycotoxins such as ochratoxin A (OTA) [12]. Multiple animal studies revealed that citrinin is primarily toxic to the kidneys, but it also affects the liver and immune system and can be carcinogenic [12]. The maximum quantity of citrinin allowed in food was recently added to European Union regulations, which indicates that this type of mycotoxin is causing a lot of concern [12].
Epoxyagroclavine belongs to a group of clavine alkaloids, the simplest ergot alkaloids, commonly produced by some Claviceps and Penicillium species [2, 10, 13]. Ergot toxicoses of humans and animals have been known for about two thousand years, with symptoms that include nausea, vomiting, and even psychiatric hallucinations, insomnia, digestive disorders, and gangrene [14]. Ergot alkaloids can be found in grains, food, and bakery products made of contaminated flour [14].
How to treat Penicillium corylophilum?
Inhalation and dermal hazards of P. corylophilum haven’t been reported so far. However, it is important to consider some risk factors such as indoor spore level and length of exposure. It is also important to consider that spores, hyphal fragments, and mycotoxins might easily spread from the crawl space to other locations [8].
Increasing knowledge of health risk factors various mold species demands a good identification practice. Cultural and microscopic characteristics of the mycelium and the conidia are a foundation for the identification of microfungal diversity. However, it is estimated that about 50% of mold identifications in literature are incorrect [8]. Therefore, proper identification should be performed by experienced mycologists at the species level.
Considering the air quality, fluctuating humidity may contribute to a high distribution of spores, hyphal fragments, and toxins [8]. Hidden moisture or mold presence in the foundation or inside the outer wall construction should be timely managed since they can significantly affect the indoor environment and contribute to the “sick building syndrome”.

Did you know?
Basements in Canada are the most affected by the Penicillium/Aspergillus mold group?! Find out more exciting mold stats and facts inside our mold statistics page.
References
- Hocking, A. D. and Pitt, I. J. (1997). Fungi and Food Spoilage. Springer, Boston, Ma. Chapter: Penicillium and related genera. pp 207-208
- McMullin, D. R. (2014). Secondary metabolites from Penicillium corylophilum isolated from damp buildings. 106 (4): 621-628
- de Senna Nunes Ales, M. et al. (2002). Isolation of Fungi in Musca domestica Linnaeus, 1758 (Diptera: Muscidae) Captured at Two Natural Breeding Grounds in the Municipality of Seropédica, Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz. 97(8): 1107-1110
- da Costa, L. G. et al. (2003). Entomopathogenic effect of Aspergillus giganteus and Penicillium corylophilum on two triatomine vectors of Chagas disease. Journal of Basic Microbiology. 43 (1): 3-7
- da Costa, L. G. et al. (1998). Pathogenic action of Penicillium species on mosquito vectors of human tropical diseases. Journal of Basic Microbiology. 38 (5-6): 337–341
- H. S. Onions and B. L. Brady (1987). Penicillium and Acremonium. Biotechnology Handbooks. Springer Science+Business Media New York. Chapter 1. pp 19.
- Lyratzopoulos, G. et al. (2002). Invasive infection due to Penicillium Species other than marneffei Journal of Infection (2002) 45: 184-207
- Bok, G. et al. (2009). Mass occurrence of Penicillium corylophilum in crawl spaces, south Sweden. Building and Environment 44: 2413–2417
- ErmiTM/Allergen. Retreated from: falaboratories.com
- Kumar, A. et al. (2017). New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier. Chapter: Secondary Metabolism and Antimicrobial Metabolites of Penicillium. pp 53
- Garcia Silva, M. et al. (2004). Antibacterial activity from Penicillium corylophilum. Microbiological Research 159: 317—322
- Čulig, B. et al. (2017). Presence of citrinin in grains and its possible health effects. African Journal of Traditional, Complementary and Alternative Medicines. 14(3):22-30
- Pannacione, D. G. (2005). Origins and significance of ergot alkaloid diversity in fungi. FEMS MIcrobiology Leters. 251(1):9-17
- Scott, P. M. (2009). Ergot alkaloids: Extent of human and animal exposure. World Mycotoxin Journal. 2(2):141-149

Get Special Gift: Industry-Standard Mold Removal Guidelines
Download the industry-standard guidelines that Mold Busters use in their own mold removal services, including news, tips and special offers:

Written by:
Jelena Somborski
Mycologist
Mold Busters
Edited by:
Dusan Sadikovic
Mycologist – MSc, PhD
Mold Busters
