Pers. 1795
What is Torula?
Torula is the fungal genus that belongs to the family Torulacae, order Pleosporales [1]. Currently, the family Torulaceae consists of four genera: Torula, Dendryphion, Neotorula, Rosticonidium, and Sporidesmioides [1,2,3]. Torula spp. are saprobes, asexual fungi [1], commonly found in both terrestrial and aquatic habitats, in temperate to tropical regions [3].

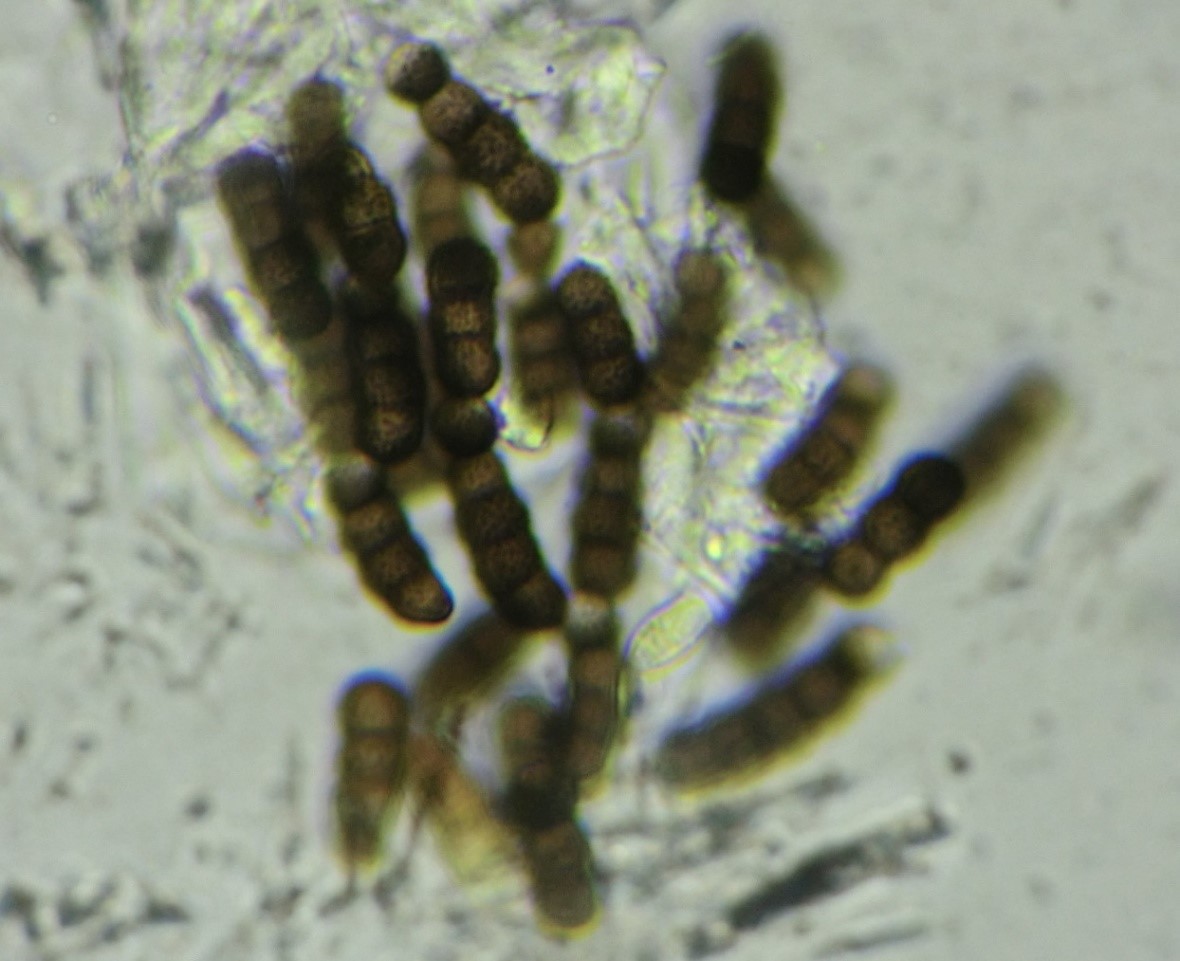
This genus was established in 1795 by Persoon [4], who wanted to group all hyphomycetes that form single-celled, dark, or subhyaline moniliform conidia (Fig. 1). T. herbarum is considered a type species and can be usually found on dead herbaceous stems, but occasionally on wood and leaves [5]. It is cosmopolitan, but most often found in temperate regions [5], producing compounds from the class of pigments that possess weak to moderate antimicrobial activity [6].
Spores of Torula species are mostly present in the air indoors, sometimes even outdoors, and are detected frequently, but in smaller amounts [7,8].
History of Torula genus
The genus has a complex history, established around 1795 to group all hyphomycetes that form single-celled, dark, or subhyaline moniliform conidia. T. herbarum was discovered in 1801 by Persoon under the name Monilia herbarum. However, eight years later Link placed it under the genus Torula, where it still belongs. Unfortunately, in 1838 the genus name was used to describe organism in beer, which later Pasteur accepted and used the name Torula for a group of beer yeast, called Torula yeast [5]. This error occurred more than 100 years ago and still makes confusion.
Synonym to Candida utilis (teleomorph Cyberlindnera jadinii) is Torula yeast, and is usually not invasive, but can cause candidemia in a patient with aplastic anemia [9]. Therefore, Torula is currently consisted of 247 species [10], with many unrecognized species within the genus. As a consequence, most of the species are not even closely related to the type species T. herbarum [1]. As another example, fossils of fungi from Baltic amber that are described in 1886 are then identified upon their morphological characteristics as a Torula globulifera and T. heteromorpha and were one of the earliest fungi specimens recognized from amber.
Recently, a study that evaluated those specimens revealed they differ from the modern Torula genus. Those species are placed to a new genus and named Casparyotorula globulifera and C. heteromorpha after founder’s name Caspary and its former genus name – Torula [5]. Luckily, recent studies were using modern molecular analyses, revealing which species truly belongs to Torula genus [1-3,11], trying to resolve this puzzle that lasts more than a century, occurred due to inadequate classification,.
How many Torula species exists?
The genus once contained more than 400 species, and by using molecular analyses many of them were replaced with other genera related or completely distinct to Torula. Currently, Torula needs to be fully evaluated, and a few studies have begun that process [1-3,11]. According to Species Fungorum [10], Torula has 247 species, but only several were verified as Torula species using molecular tools [3]. In recent phylogenetic research regarding relationships within the Torulaceae family [11], five species were placed placed in Torula genus: T. herbarum, T. monilis, as well as 3 new species: Torula ficus, Torula holdandica, and Torula masonii. Furthermore, seven new species have been recently added to the genus: T. mackenziei, T. chromolaenae, T. chiangmaiensis, T. pluriseptata, T. camporesii, and Torula gaodangensis [1–3].
What do Torula species look like?
Torula species form velvety, dark brown to black colonies with mostly immersed mycelia. Species produce conidia that are globose to subglobose in shape, brown in color with pale brown apex, smooth to verrucose in texture. Conidia are usually composed of four cells (phragmoconidia), but can go up to ten (Fig. 1). Those conidia are borne in branched chains, starting from conidiogenous cells or one brown supporting cell, and its apical cells have the ability to become conidiogenous, actually to form new conidia from its apex [3,11].
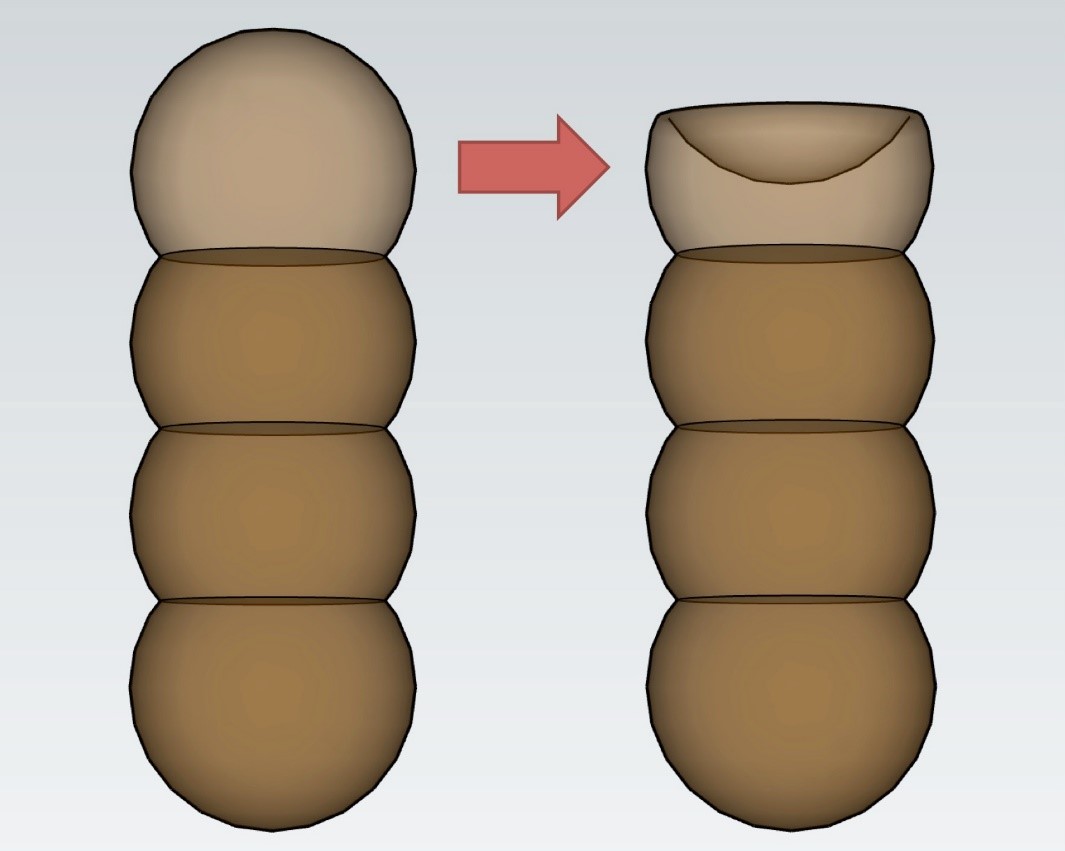
Conidiogenous cells can be terminal or lateral, have thickened and heavily melanized wall on the base, or thin wall that frequently collapses and becomes coronate on the apex (Fig. 2) [3]. Representative species T. herbarum forms a pale olive-gray colony on PDA and MEA media, which cover the whole dish after two weeks on 25°C (77°F). It forms subglobose conidia mostly 3-septate 16–17 x 6 µm in size, or 4–septate conidia, 22–24 x 6–7 µm in size [11].
Where can be Torula found?
Torula spores are found frequently, but in a smaller amount, representing only a few percent of the total tested aeromycoflora [7]. Spores are mostly present in the air indoors, but sometimes even outdoors. In Istanbul, Turkey, T. herbarum was detected in smaller percent (1-2%) from indoor air in all six examined districts [8]. Part of libraries where old books, magazines, and manuscripts were stored, Torula together with Gibberella was a dominant genus [12]. A probable source of spores could be paper dust, wooden materials, and the damp wall of the library. Also, Torula spores are present in the air inside of a factory, suggesting damp wood materials are the main source of their spores [13]. Only one sample was isolated from hostel indoor air in Nigeria [14], and one sample was from a classroom in India [15].
Interestingly, studies from Cordoba, Spain, showed spores belonging to the Torula genus were alongside other fungal genera: Cladosporium, Coprinus, and Alternaria one of the most abundant spores present in indoor and outdoor air of the daily crowded train [16]. Outdoors, Torula was mostly detected in the air around crop fields after harvest, suggesting plant debris is a major source of these fungi [13]. T. herbarum is found on the apple fruit surface, and together with other fungi form a fungal complex that causes apple sooty blotch [17]. Species are also detected on the surface of Solanum nigrum leaves, living as an endophyte [18].
Torula produces metabolites
T. herbarum is reported to produce herbarin, dehyroherbarin, and o-methyl herbarin, a class of pigments that has a weak to moderate antimicrobial activity [6], while dehydroherbarin has high antiviral activity against Hepatitis A virus [19]. All compounds showed high toxicity towards the breast cancer cell line [19]. Recent discoveries isolated a new heptaketide named herbarone from T. herbarum originated from the internal organs of sea hare [20].

Did you know?
The #1 toxic mold type found in homes is the Penicillium/Aspergillus mold group?! Find out more exciting mold stats and facts inside our mold statistics page.
References
- Li J-F, Phookamsak R, Jeewon R, Bhat DJ, Mapook A, Camporesi E, Shang Q-J, Chukeatirote E, Bahkali AH, Hyde KD (2018). Molecular taxonomy and morphological characterization reveal new species and new host records of Torula species (Torulaceae, Pleosporales). Mycological Progress, 16:447–461.
- Su X-J, Luo Z-L, Jeewon R, Bhat DJ, Bao D-F, Li W-L, Hao Y-E, Su H-Y, Hyde KD (2018). Morphology and multigene phylogeny reveal new genus and species of Torulaceae from freshwater habitats in northwestern Yunnan, China. Mycological Progress, 17:531–545.
- Hyde KD, Dong Y, Phookamsak R, Jeewon R, Bhat DJ et al. (2020). Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity, 100:5–277.
- Retrieved from (december, 2021): http://www.indexfungorum.org
- Kettunen E, Grabenhorst H, Gröhn C, Dörfelt H, Sadowski E-M, Rikkinen J, Schmidt AR (2015). The enigmatic hyphomycete Torula sensu Caspary revisited. Review of Palaeobotany and Palynology, 219:183–193.
- Wang L, Dong JY, Song HC, Shen KZ, Wang LM, Sun R, Wang CR, Li GH, Li L, Zhang KQ (2008). Screening and isolation of antibacterial activities of the fermentative extracts of freshwater fungi from Yunnan Province, China. Annals of Microbiology, 58(4):579-584.
- Grinn-Gofron A, Bosiacka B (2015). Effects of meteorological factors on the composition of selected fungal spores in the air. Aerobiologia, 31:63–72.
- Colakoglu G (2004). Indoor and Outdoor Mycoflora in the Different Districts of the City of Istanbul (Turkey). Indoor Built Environment, 13:91–100.
- Treguier P, David M, Gargala G, Camus V, Stamatoullas A, Menard A-L, Lenain P, Contentin N, Lemasle E, Lanic H, Tilly H, Jardin F, Lepretre S (2018). Cyberlindnera jadinii (teleomorph Candida utilis) candidaemia in a patient with aplastic anaemia: a case report. JMM Case Reports, 5(8):e005160.
- Retrieved from (december, 2021): http://www.speciesfungorum.org
- Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernandez-Restrepo M, Jaklitsch WM, Lebrun M-H, Schumacher RK, Stielow JB, van der Linde EJ, Vilcane J, Voglmayr H, Wood AR (2015). The Genera of Fungi – fixing the application of the type species of generic names–G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus, 6(1):163–198.
- Wu D, Zhang Y, Qin W, Zhao C, Li J, Hou Y, Xiong J, Li A, Gao R (2021). Seasonal structural characteristics of indoor airborne fungi in library rooms by culturing and high-throughput sequencing. Building and Environment, 206:108368.
- Karmakar B, SenGupta K, Kaur A, Roy A, Bhattacharya SG (2020). Fungal bio‑aerosol in multiple micro‑environments from eastern India: source, distribution, and health hazards. SN Applied Sciences, 2:565.
- Durugbo EU, Kajero AO, Omoregie EI, Oyejide NE (2013). A survey of outdoor and indoor airborne fungal spora in the Redemption City, Ogun State, south-western Nigeria. Aerobiologia, 29:201–216.
- Karmakar P, Das U, Das P, Saha AK (2020). Airborne fungal spore concentration in some selected indoor and outdoor sites: Threats of respiratory problems. Tropical Plant Research, 7(1): 94–100.
- Garcia-Mozo H, Lopez-Orozco R, Canalejo C, Oteros J (2020). Indoor biological particles in a train: comparative analysis with outdoor atmosphere. Aerobiologia (2020) 36:481–492.
- Grabowski M (2007). The study of new fungus species causing apple sooty blotch.
- Nayak BK (2018). Enumeration of phylloplane and endophytic fungi from medicinal plant, Solanum nigrum by two different techniques. International Journal of Chemical Concepts, 4(1):01-06.
- Osmana ME, El-Beih AA, Khatab O-KH, Moghannem SAM, Abdullah NH (2018). Production of herbarin and dehydroherbarin by endophytic Chaetosphaeronema (KY321184) isolated from Nepeta septemcrenata and evaluation of their bioactivities.
- Geng W-L, Wang X-Y, Kurtan T, Mandi A, Tang H, Schulz B, Sun P, Zhang W (2012). Herbarone, a Rearranged Heptaketide Derivative from the Sea Hare Associated Fungus Torula herbarum. Journal of Natural Products, 75(10):1828–1832.

Get Special Gift: Industry-Standard Mold Removal Guidelines
Download the industry-standard guidelines that Mold Busters use in their own mold removal services, including news, tips and special offers:

Written by:
Aleksandra Zebeljan
Mycologist
Mold Busters
Edited by:
Dusan Sadikovic
Mycologist – MSc, PhD
Mold Busters
